Research
① [Drug Discovery Research]
Epigenetics × Drug Discovery → Development of Novel Therapeutics
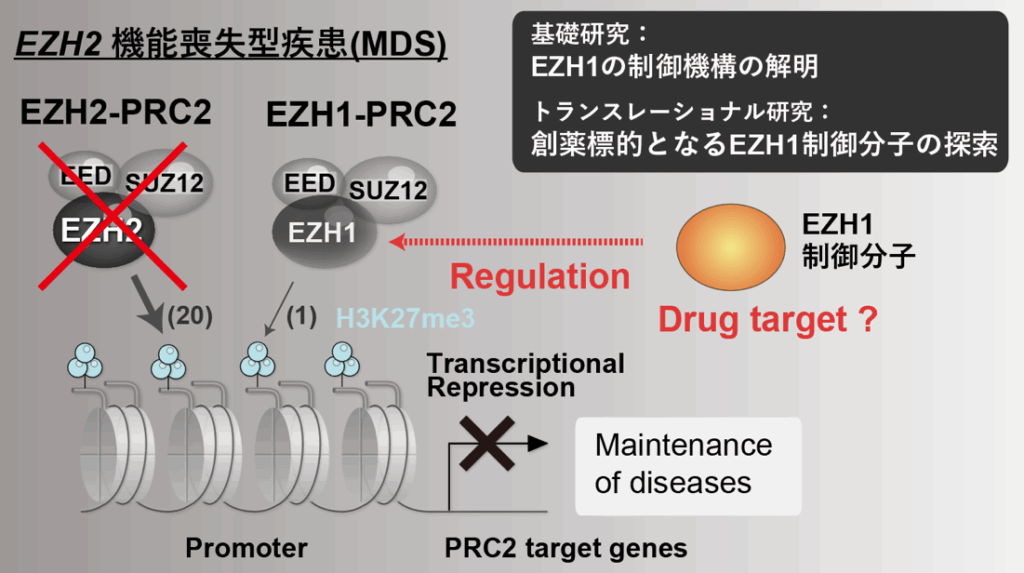
The enzymatic subunit EZH2 of the Polycomb Repressive Complex 2 (PRC2) is the primary enzyme responsible for the transcriptionally repressive histone modification H3K27me3. Dysregulation of this enzyme is closely associated with severe diseases such as myelodysplastic syndrome (MDS), a type of leukemia. We have established a mouse model of EZH2 loss-of-function MDS and discovered that EZH1 is essential for the maintenance of the disease. Interestingly, in the wild type, loss of EZH1 has only limited effects, making EZH1 and its regulatory mechanisms promising drug targets with potentially minimal side effects. We are currently elucidating the detailed regulatory mechanisms of EZH1 through BioID proteomic analysis and CRISPR screening, aiming to apply the identified regulatory molecules to drug discovery.
At present, our research primarily focuses on PRC2-related cancers, particularly MDS. However, in the future, we plan to introduce disease-specific iPS cell models to expand into a broader range of disease areas, including major social health challenges such as mental disorders (e.g., depression) and developmental disorders (e.g., ASD, ADHD).
② [Aging Research]
Epigenetics × Aging Research → Extension of Healthy Lifespan
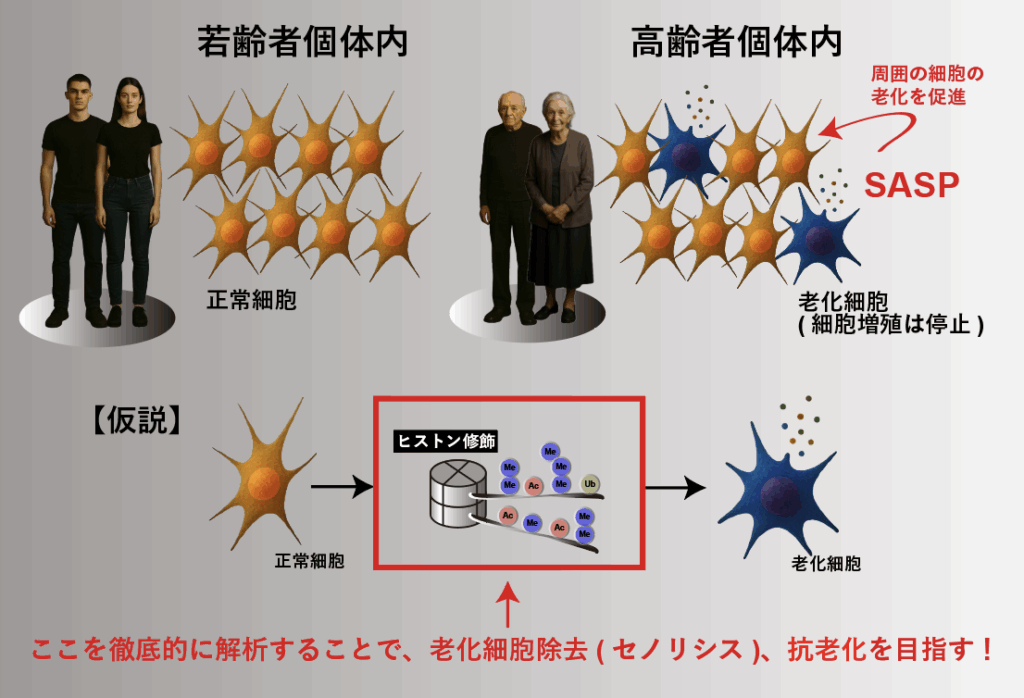
Aging has long been regarded as an “inevitable natural phenomenon,” but in recent years it is being redefined as a “treatable disease.” Senescent cells cease proliferation and secrete inflammatory factors known as the senescence-associated secretory phenotype (SASP), which induce chronic inflammation and propagate aging in surrounding tissues, thereby promoting the onset of age-related diseases. In our laboratory, we analyze histone modifications in senescent and normal cells with the aim of developing novel intervention strategies, such as senolytic drugs that selectively eliminate senescent cells and SASP modulation therapies. The removal of senescent cells is expected to contribute to the prevention of all aging-related diseases, including cancer.
We also focus on “epigenetic memory,” the persistent marks of chromatin modifications. By analyzing histone modifications accumulated in the cells of aged individuals, we aim to elucidate the epigenetic memory associated with aging, thereby building a foundation for a fundamental understanding of aging control and paving the way toward extending healthy lifespan to its maximum potential.
③ [Food Science]
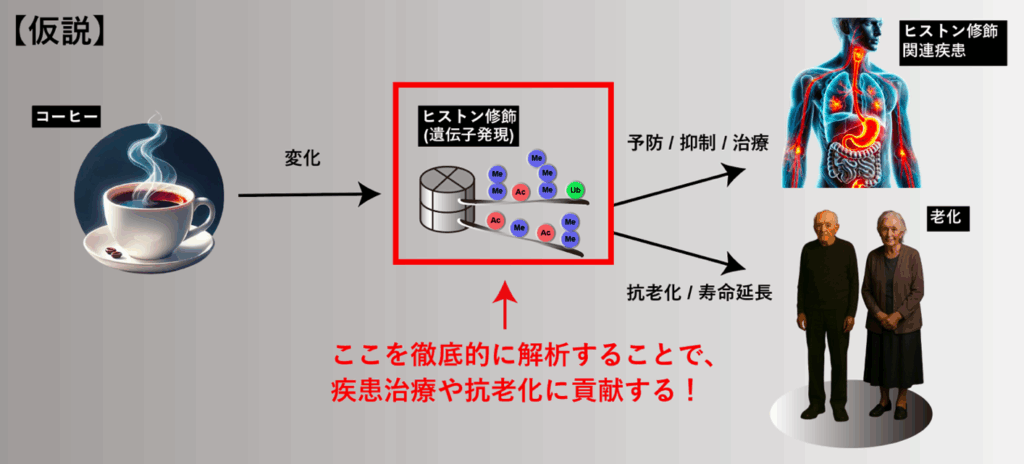
Epigenetics × Food Science → Extension of Healthy Lifespan
Epidemiological studies have reported that coffee consumption is associated with various health benefits, including increased longevity, reduced cardiovascular disease risk, and prevention of diabetes. However, few studies have clarified the contribution of epigenetics—specifically histone modifications—to these coffee-related effects. Through our histone modification analyses, we have discovered that coffee treatment induces significant changes in numerous histone modifications. Some of these modifications have been reported to be associated with health and lifespan, suggesting that histone modification changes may play a crucial role in the beneficial effects of coffee. We aim to develop this finding into preventive medical strategies—such as combining coffee-related interventions with histone modification–targeted drugs—to enhance therapeutic outcomes and extend healthy lifespan.